Recent Research on Postnatal Neuronal Development in Cell Reports and Molecular Psychiatry
Rui Peixoto, PhD (Assistant Professor of Psychiatry), is an expert in developmental neuroscience and investigates abnormalities in cortical and striatal development associated with autism spectrum disorder. He is senior author of recent papers published in Cell Reports and Molecular Psychiatry.
“Our recent findings provide new insight into how the striatum and basal ganglia shape the maturation of prefrontal cortical circuits,” said Dr. Peixoto. “We show that manipulations of striatal neurons profoundly alter cortical activity and disrupt the developmental trajectory of synaptic connectivity, particularly the maturation of cortical inhibition. In complementary work, we identified parvalbumin-expressing interneuron dysfunction as an early pathophysiological feature in a mouse model of autism, which also exhibits early deficits in striatal activity and connectivity. Together, these studies suggest that striatal dysfunction contributes to abnormal cortical maturation and that altered basal ganglia output during postnatal development may confer a vulnerability of cortical inhibition, increasing susceptibility to neurodevelopmental and psychiatric disorders.”
Striatal output Regulates the Postnatal Maturation of Cortical Circuits
The dorsomedial prefrontal cortex and basal ganglia are tightly interconnected through cortico- basal ganglia-thalamocortical loops that that support motor control and cognition, and which undergo extensive refinement during postnatal development. While the role of cortical activity in shaping striatal circuit maturation is well established, the extent to which the basal ganglia regulate dorsomedial prefrontal cortex development remains unclear.
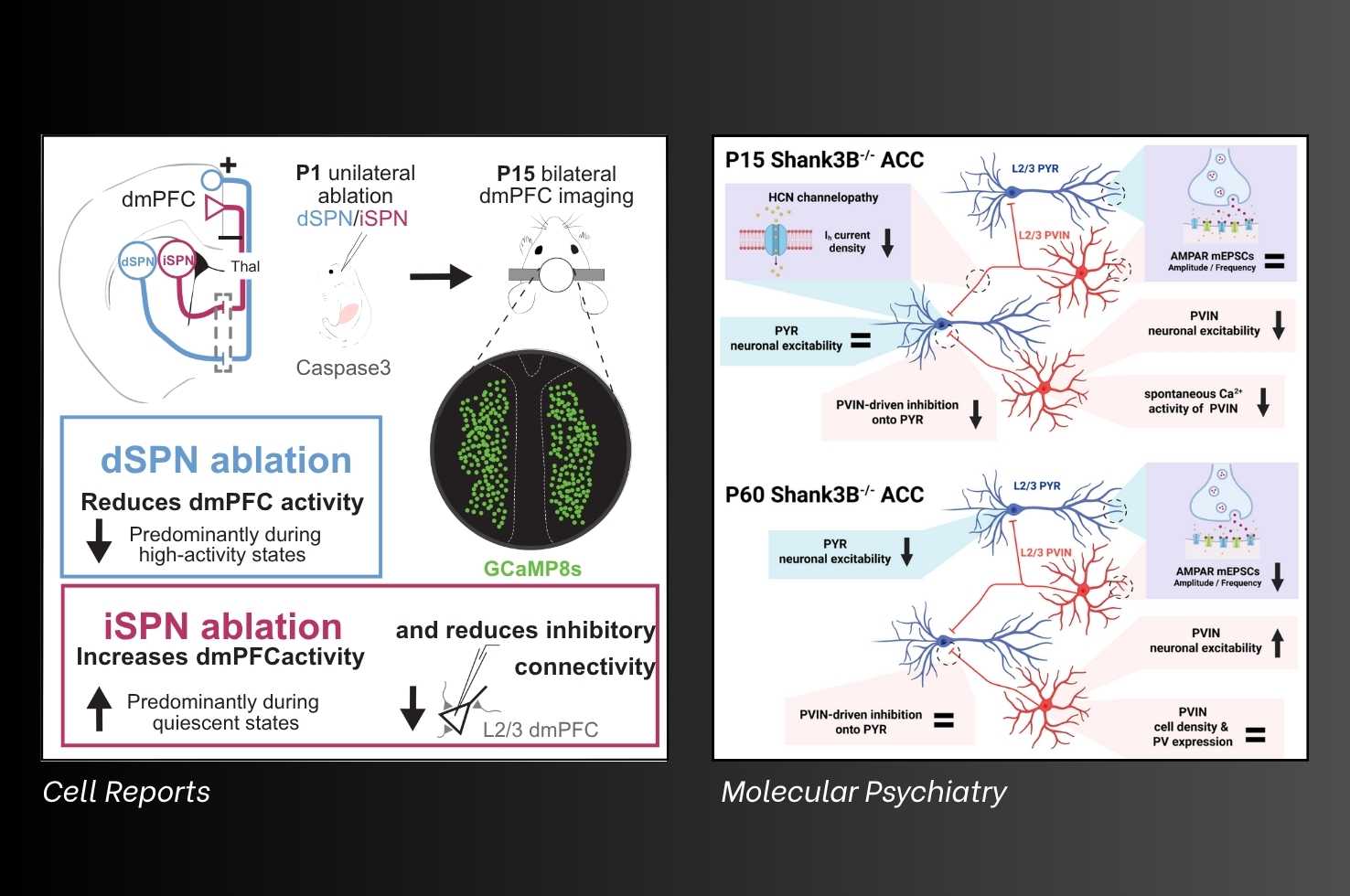
A team of scientists led by Dr. Peixoto, and including Michael Janeček, BS (Center for Neuroscience at the University of Pittsburgh graduate student), Tara Deemyad, MD, PhD (former University of Pittsburgh postdoctoral scholar), Kyle Ketchesin, PhD (Assistant Professor of Psychiatry), examined whether early striatal output influences the maturation of dorsomedial prefrontal cortex activity and connectivity. Targeted ablation of direct or indirect pathway spiny projection neurons during the first two postnatal weeks induced bidirectional changes in dorsomedial prefrontal cortex neural activity akin to basal ganglia modulation of cortical dynamics observed in mature circuits. These manipulations also disrupted synaptic maturation of layer 2/3 pyramidal neurons, shifting the balance between excitation and inhibition.
Janeček M, Deemyad T, Shih YC, Valle V, D’Agostino A, Matarazzo M, Perez MS, Ketchesin KD, da Silva S, Peixoto RT.
Cell Reports, Volume 44, Issue 9, 116187.
Early Postnatal Dysfunction of ACC PV Interneurons in Shank3B−/− Mice
Anterior cingulate cortex dysfunction is implicated in the cognitive and social deficits of autism spectrum disorder, yet the developmental trajectory and mechanisms driving these circuit abnormalities remain poorly understood.
Dr. Peixoto and Yi-Chun Shih, MS (graduate student, Center for Neuroscience at the University of Pittsburgh), investigated the postnatal development of glutamatergic connectivity and intrinsic excitability in layer 2/3 pyramidal neurons (PYRs) and parvalbumin-expressing interneurons (PVINs) in the anterior cingulate cortex of Shank3B−/− mice.
They found that PVINs exhibit reduced excitability and in vivo hypoactivity as early as postnatal day 15, despite receiving normal levels of glutamatergic input. This early PVIN hypoexcitability was linked to decreased feedforward inhibition from the mediodorsal thalamus and reduced hyperpolarization-activated currents mediated by hyperpolarization-activated cyclic nucleotide gated (HCN) channels. In contrast, pyramidal neurons displayed normal excitability and synaptic input at this stage but already showed diminished HCN-mediated currents, pointing to an early, shared emergence of HCN channel dysfunction in both PVINs and PYRs. By adulthood, both cell types exhibited marked phenotypic changes, with reduced glutamatergic input and divergent alterations in excitability. Together, these findings link early PVIN deficits in Shank3-related disorders to HCN dysfunction and underscore the importance of studying early developmental mechanisms to understand the pathogenesis of cortical circuit dysfunction in autism spectrum disorders.
Shih YC, Nelson L, Janeček M, Matarazzo M, D’Agostino A, Peixoto R.
Molecular Psychiatry, (2025). https://doi.org/10.1038/s41380-025-03114-w
